Someone asked me some questions the other day about magnetite. He knew it was a magnetic mineral but wondered about why it was magnetic (it came up because I had mentioned that I led students to some old magnetite mines that day).
I didn't really know exactly why magnetite was magnetic (I had some vague memory that it might have something to do with the different oxidation states of iron in the mineral, but didn't remember beyond that). So I did some research.
 Before we talk about magnetite, let's discuss another oxide of iron called hematite (Fe2O3). Hematite is a common rusty-red mineral and we've all seen it in the form of rusted iron.
Before we talk about magnetite, let's discuss another oxide of iron called hematite (Fe2O3). Hematite is a common rusty-red mineral and we've all seen it in the form of rusted iron.Iron can occur in two oxidation states - iron(II) or ferrous iron (Fe2+) and iron(III) or ferric iron (Fe3+). The iron atoms in hematite occur as ferric iron (oxygen has an oxidation state of -2 so two Fe3+ and three O2- balance each other out).
Now it gets more complicated. Iron, number 26 on the periodic table, has 26 protons (positively charged particles in the nucleus) and is orbited by 26 negatively charged electrons (when the atom is electrically neutral). The electrons occupy specific orbitals and, in iron, they fill up in the following way:
Fe: 1s22s22p63s23p63d64s2
Fe: 1s22s22p63s23p63d64s2
where 1s, 2s, 3s, 4s, 2p, 3p, and 3d all represent specific orbitals for the electrons and the superscripts represent the number of electrons in each (a bit more complicated than the very simple Bohr model of the atom most of you are probably familiar with unless you've had some chemistry). Note there are 26 electrons in all 7 orbitals (s, p, & d orbitals have the following shapes):
Electrons have a property called spin which describes their angular momentum. Electron spin is described as either up or down and as electrons fill orbitals, they do so in pairs where one electron spins upward and the other spins downward. One interesting thing about iron, however, is that it has four unpaired electrons in its 3d orbital.
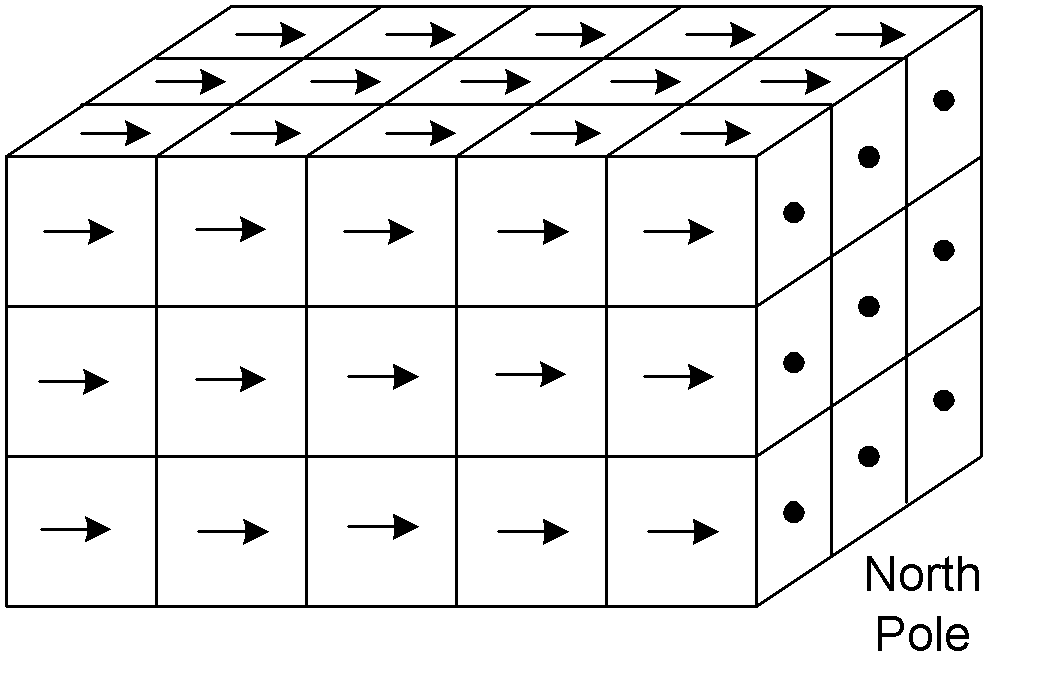
Materials, like iron, which exhibit this property are called ferromagnetic. Wrap a coil of copper wire around this block of iron, run an electrical current through it, and the electrical current will form a magnetic field (that's why it's called electromagnetism) and all the domains in the iron will line up and turn it into a magnet (an electromagnet). When the current is turned off, however, the domains go back to random orientation and the iron demagnetizes. This also explains why magnets stick to iron and not, for example, to aluminum. The magnetic field of the magnet lines up the domains in the iron and it sticks. Below shows magnetized Fe.
Ferrric iron found in hematite, however, has the following electron configuration:
Fe3+: 1s22s22p63s23p63d54s0
 Given its charge of +3, it's going to be short 3 electrons which come out of the 3d and 4s orbitals. Now all 5 of the electrons in the 3d orbital are unpaired. Hematite crystallizes in a hexagonal structure (at left) such that atoms in one plane are aligned and in the next plane they're aligned but in an opposite direction. These spins cancel each other out making hematite antiferromagnetic.
Given its charge of +3, it's going to be short 3 electrons which come out of the 3d and 4s orbitals. Now all 5 of the electrons in the 3d orbital are unpaired. Hematite crystallizes in a hexagonal structure (at left) such that atoms in one plane are aligned and in the next plane they're aligned but in an opposite direction. These spins cancel each other out making hematite antiferromagnetic.A magnet will not stick to a chunk of pure hematite.
Two asides... In reality, above -10° C, Fe atoms in hematite are slightly tilted making it weakly ferromagnetic but below -10° C the Fe lines up better and it becomes truly antiferromagnetic. Also note there is some stuff sold as "magnetic hematite" but that's an advertising term for a synthetic material, it's not the natural mineral hematite.
Now we can discuss magnetite. Magnetite (Fe3O4) is also a common ore of iron but, unlike hematite, it contains both oxidation states of iron so can be called iron(II,III) oxide or ferrous-ferric oxide and the formula can also be written as FeO • Fe2O3. where the FeO is ferrous iron (Fe2+) and the Fe2O3 is ferric iron (Fe3+).
We've already discussed electron configuration in ferric iron (Fe3+). What about ferrous iron (Fe2+)?
Fe2+: 1s22s22p63s23p63d64s0
Given its charge of +2, it is obviously going to be short 2 electrons from Fe. As you can see, they come out of the 4s orbitals and, like regular Fe, it still has 4 unpaired electrons in the 3d orbital (meaning it's also ferromagnetic).
What makes magnetite different from hematite, and strongly magnetic like regular iron, is that it has a different crystalline structure. The ferric iron domains cancel each other out but the ferrous iron domains can be lined up with a magnet making the mineral magnetic (I'm pretty sure that's correct, but don't cite me in a paper if you're a student!).
Magnetite iron ore (with magnet) from Hudson Highlands, NY
Magnetite's easy to recognize in the field typically occurring as a black, fairly-dense, crystalline mineral that's strongly magnetic.
Some varieties of magnetite act as a permanent magnet and can pick up paper clips and even nails. These are called lodestone which meant 'course stone' or 'leading stone' in Middle English because they were used to construct the earliest compasses.
So why is lodestone permanently magnetized? One idea is that it's the result of near-surface iron ore deposits being struck by lightning and lining up the magnetic domains (see this paper for technical details).







No comments:
Post a Comment